
 After an evening of 5-10 degree temperatures and following some heavy snow, the valleys in the SW VA Blue Ridge had a magnificent frosting event. The crystals of frost grew large and heaved themselves into upright positions. I found these on top of my car.
After an evening of 5-10 degree temperatures and following some heavy snow, the valleys in the SW VA Blue Ridge had a magnificent frosting event. The crystals of frost grew large and heaved themselves into upright positions. I found these on top of my car. 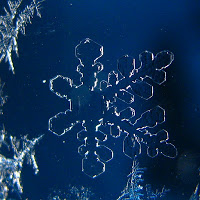 some of the frost growth presented itself as perfect crystals...identical to individual snowflakes. There was one significant difference...size. These "frost flakes" were twice the size of the snowflakes I witnessed a couple of weeks ago. I estimate the frost to be between 1/4" -1/2" in total crystal width. The snowflakes were tiny...always less than 1/4" wide.
some of the frost growth presented itself as perfect crystals...identical to individual snowflakes. There was one significant difference...size. These "frost flakes" were twice the size of the snowflakes I witnessed a couple of weeks ago. I estimate the frost to be between 1/4" -1/2" in total crystal width. The snowflakes were tiny...always less than 1/4" wide.  And so, I am left with questions:
And so, I am left with questions:- Why is there such a similarity in crystal structure and appearance and yet a difference in size?
- Were these frost crystals permitted a greater amount of time for growth (and thus grew larger)?
- Could they have grown at a slower rate under sustained conditions?
- Given that water is a mineral, is it likely that its crystal growth behaves similarly to that of other minerals like sugar, salt, emerald, quartz, pyrite, to name a few?
- What are the conditions for optimal water crystal growth anyway??













